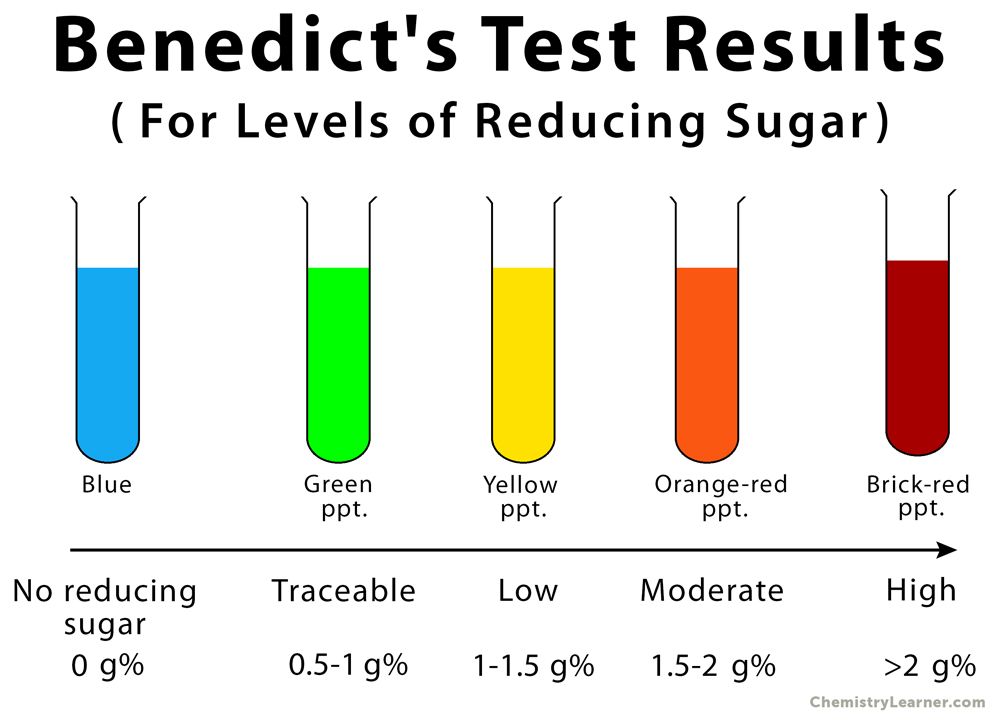
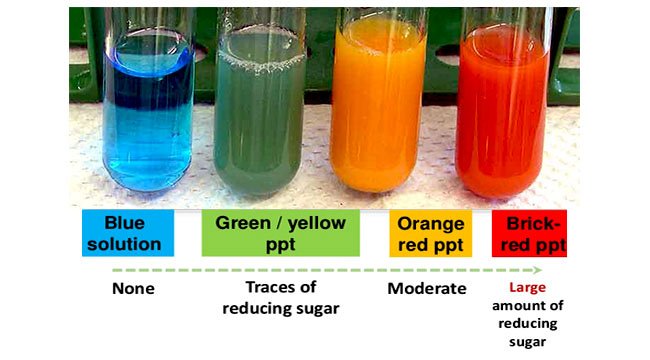
Water Starch Then test for sugars with benedicts. In presence of simple sugars the blue solution changes color to either green yellow or brick-red depending on the amount of sugar.

Benedict S Test Reagent Preparation Principle Procedure Reaction
In this article we will learn about the benedicts test in detail.

. Benedicts test for glucose is a chemical test that can be used to determine whether or not an sample contains reducing sugars. Benedicts Test is used to test for simple carbohydrates. Some sugars such as glucose are called reducing sugars because they are.
Benedicts Solution or one of the many variants that evolved over the years was used as the reagent of choice for measuring sugar content for more than 50 years. What is Benedicts Test. Bring the solution to heat in a boiling water bath for approximately five minutes.
Put about 10 drops of Benedicts reagent in the test tube. Take 5ml of Benedicts solution in to a boiling tube. It is a clear blue solution of sodium and copper salts.
Benedicts test for glucose reagent also known as Benedicts solution is a complicated mixture of. The test is based on Benedicts reagent also known as Benedicts solution. Benedicts solution is used to test for simple sugars such as glucose.
Benedicts Reagent Benedicts Solution Benedicts reagent is the solution used in Benedicts test to detect simple sugars such as glucose. Mix and heat for 5 minutes. Reducing sugars are those sugars that have free aldose or ketose groups capable of.
Which of the following would be an example of a negative control. Group of answer choices A solution containing sugar but no Benedicts solution. Benedicts test is utilized to test for carbohydrates and non-reducing or reducing sugar.
Benedicts test is a chemical test that is used to test for the presence of reduced sugars within an analytical test. A chemical reaction used to test for the presence of an aldehyde in an unknown frequently a carbohydrate. Benedicts Test is a chemical analytical method used for the detection of reducing sugar in a solution.
In the presence of simple sugars the blue solution changes color to green yellow and brick-red depending on the amount of sugar. To perform the test one adds Benedicts solution a blue solution containing Cu 2 to the material to be tested. Place inside the test tube 1 ml of sample.
If an aldehyde is present a brick red Cu 2 O precipitate is formed. Benedicts Test is a qualitative test often used for the differentiation of carbohydrates saccharidessugars into reducing and non-reducing types. Thus simple carbohydrates that contain an aldehyde or free ketone functional group are detected using this test.
Benedicts answer can be utilized to test for the presence of glucose in urine. As a result this test can identify simple carbohydrates having a free ketone or aldehyde functional group. Urine sample is collected in to a clean ontainer.
The test is basing itself upon Benedicts Reagent also called Benedicts solution which is a complex mix. Benedicts solution can be used to test for the presence of glucose in urine. Add 8 drops of urine in to the tube.
The Benedicts test procedure includes the following. Approximately 1 ml of sample is placed into a clean test tube. It is a clear blue solution of sodium and copper salts.
A solution containing a sample and the Benedicts solution. Benedicts solution is used as a qualitative test for the presence of sugars in solution. Method 1Mix smalls amount of each food sample ie Egg lumen cylindrical piece of potato tuber bread crump and crisps in.
Benedicts reagent often called Benedicts qualitative solution or Benedicts solution is a chemical reagent and complex mixture of sodium carbonate sodium citrate and copperII sulfate pentahydrate. It is often used in place of Fehlings solution to detect the presence of reducing sugarsThe presence of other reducing substances also gives a positive result. It is a bright blue solution that is prepared by mixing copper sulfate pentahydrate sodium carbonate and sodium citrate in distilled water.
The Benedicts test identifies reducing sugars monosaccharides and some disaccharides which have free ketone or aldehyde functional groups. The solution is then heated in a boiling water bath for 3-5 minutes. Observe for color change in the solution of test tubes or precipitate formation.
5H 2 O sodium citrate Na 3 C 6 H 5 O 7 and sodium carbonate Na 2 CO 3 in distilled water 4. It is a bright blue solution prepared by mixing copper sulfate pentahydrate CuSO 4. Therefore simple carbohydrates containing a free ketone or aldehyde functional group can be identified with this test.
2 ml 10 drops of Benedicts reagent CuSO4 is placed in the test tube. It was the most common test for diabetes and was the standard procedure for virtually all clinical laboratories. October 2 2016 by Admin 2 Comments.
Benedicts test Procedure. Benedicts test is a chemical test that can be used to check for the presence of reducing sugars in a given analyte. The Benedicts test separates reducing sugars monosaccharides and some disaccharides which have free ketone or aldehyde.
Benedicts solution is most commonly used for testing if sugars are present in any enzyme or solution that contains starch. Benedicts reagent also known as benedicts solution is used in Benedicts test for detecting simple sugars such as glucose. Saul Roseman remembers that all inductees into the army during World.
Remove from the heat and allow it to cool. Benedicts solution is used to test for simple sugars such as glucose. Get a clean test tube.

Benedict S Test Principle Procedure And Uses Dewwool
Benedict S Test Principle Requirements Procedure And Result Interpretation Online Science Notes
Benedict S Test Principle Preparation Procedure And Result Interpretation

Food Test 2 Benedict S Test For Reducing Sugars Biology Notes For Igcse 2014
0 Comments